Paul Szumita joins to highlight a June Trial of the Week “Drotrecogin alfa (activated) in adults with septic shock” aka PROWESS-SHOCK, published in the New England Journal of Medicine in 2012.
To fully understand the discussion regarding drotrecogin alfa, Paul and I review the mechanism of action and its proposed action in the treatment of sepsis. Then we review earlier studies and the controversy behind the FDA approval process. Finally, we go into detail on what Clinical Pharmacists were tasked with doing at the time based on treatment protocols or hospital restrictions.
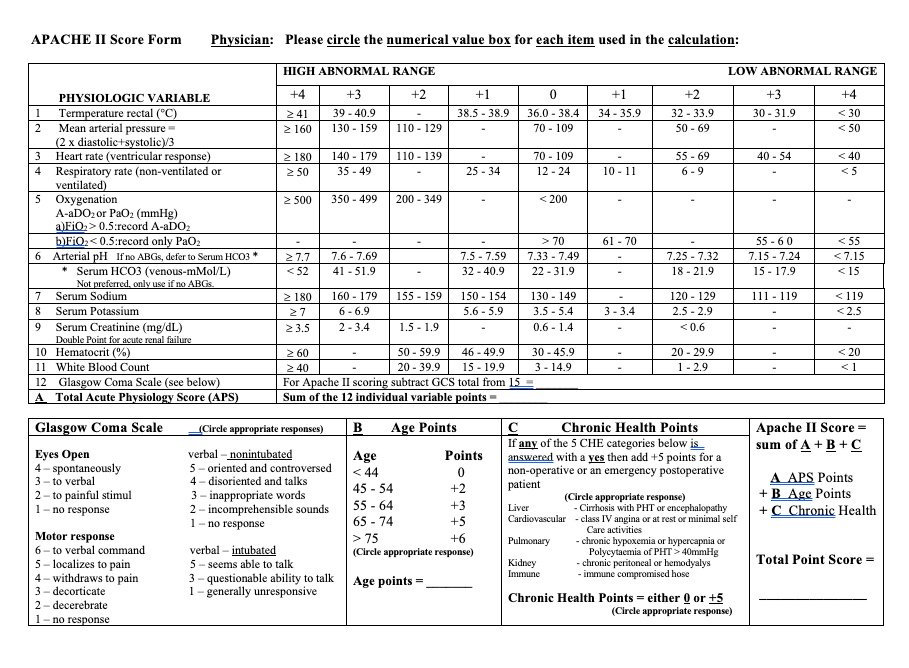
Then we dive into the Trial of the Week, discussing the study design and results. What was the mood in ICUs and Pharmacy Departments after this publication? How much did our sepsis care improve from PROWESS to PROWESS-SHOCK? What lessons did we learn with the Xigris FDA approval process? Is there a patient population that could still benefit from drotrecogin alfa treatment? Plus, trial fun facts, issues with calculating APACHE II scores, and so much more.
As discussed on the episode, below are screenshots of a sample protocol and APACHE II calculation sheet.



Leave a comment