The FDA Cellular, Tissue, and Gene Therapies Advisory Committee recently held a meeting discussing the supplemental Biologics License Application of andexanet alfa.
This committee meeting was livestreamed on Youtube, with a link available to rewatch the entire meeting here
Using slides and quotes from the FDA, the CTGT committee members, and AstraZeneca, I recap the findings and discussion from the meeting to help give the audience a better idea of the FDA expectations.
Below are some notable slides from the presentation that emphasize different points highlighted throughout the episode.

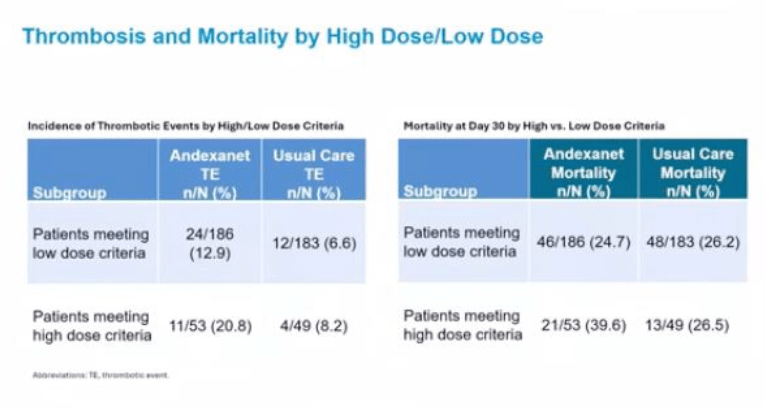
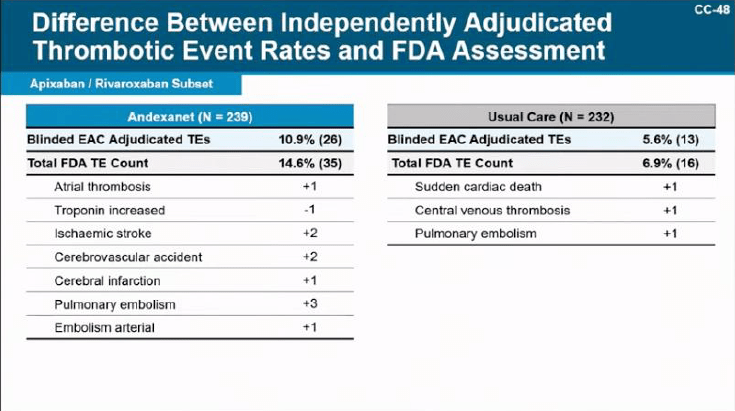
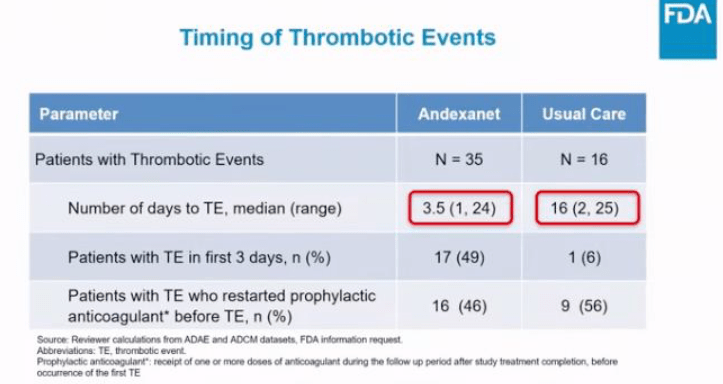

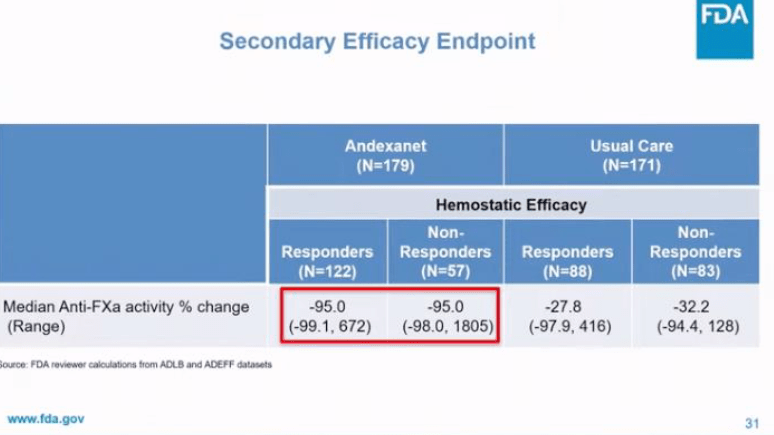

Leave a comment